
Soil pH plays a critical role in crop health and yield, yet many fields in Western Canada face the challenges of acidic soils. When soil pH drops below 6, several key issues arise, directly impacting crop productivity.
The diagram below illustrates how soil pH affects the availability of key nutrients and microbial activity. It highlights the optimal pH range for crop growth and the consequences of falling outside this range.
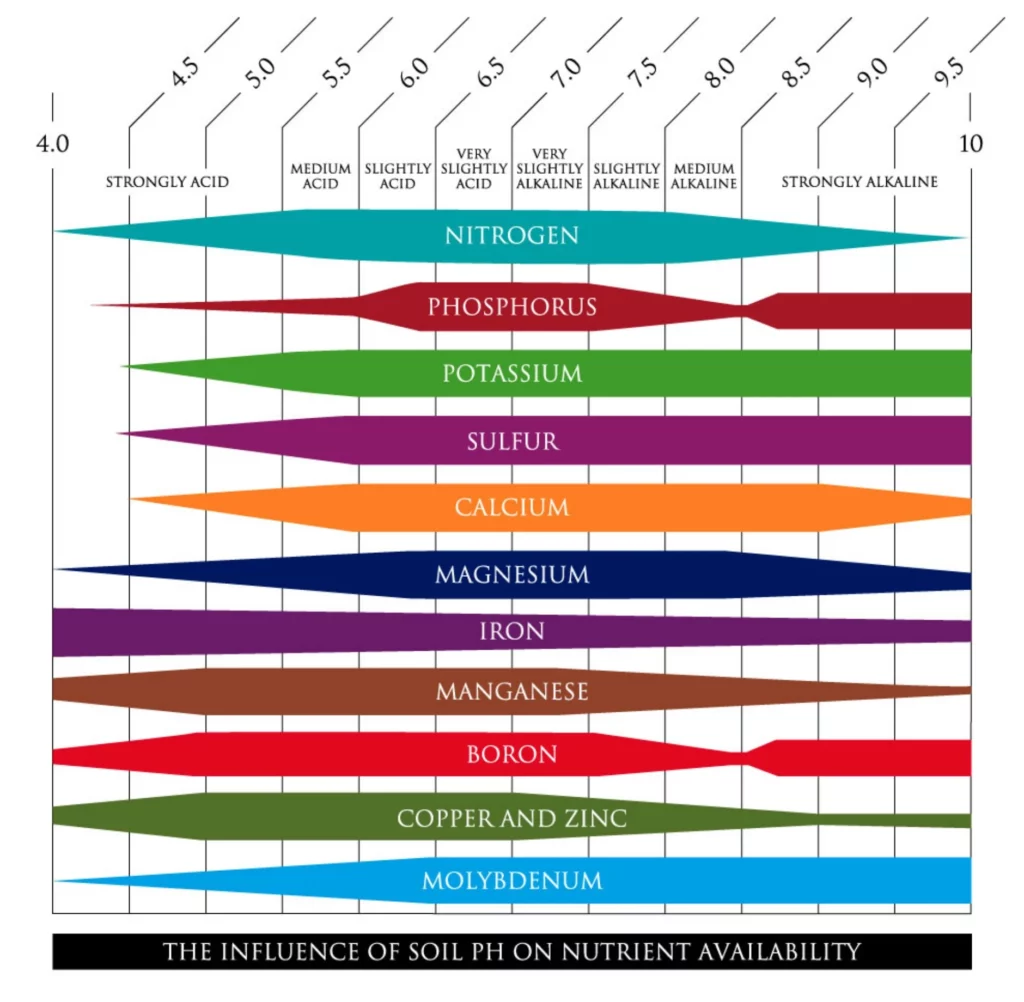
Nutrients like nitrogen (N), phosphorus (P), and potassium (K)—vital for crop growth—are most available in a neutral pH range (6-7). As soil pH decreases below 6, these essential nutrients become insoluble and unavailable for plant uptake. Conversely, elements like iron (Fe) and manganese (Mn) remain soluble, but their excess availability can further complicate nutrient imbalances. Acidic soils also cause essential cations like calcium (Ca²⁺), magnesium (Mg²⁺), and potassium (K⁺) to leach away when paired with bicarbonate ions. The result? Crops struggle to meet their nutritional needs, leading to lower yields and poor quality.
Acidic soils lead to increased solubility of aluminum (Al), which becomes toxic to crops. Aluminum ions displace calcium (Ca²⁺) in root cells, weakening cell walls and reducing calcium uptake. Furthermore, aluminum binds with phosphorus (P) in the soil, rendering it unavailable to plants. Simultaneously, manganese (Mn) in excess triggers oxidative stress in crops, damaging cellular function and further hindering growth.
Soil microbes drive nutrient cycling and organic matter decomposition, but low pH disrupts their activity. Bacteria, crucial for processes like nitrogen fixation, experience a significant decline in acidic conditions. While fungi remain active across a broader pH range, the overall microbial diversity and efficiency of nutrient cycling decrease, impacting soil health and fertility.
Learn more about how H-Start can help you address low soil pH on your farm. H-Start improves nutrient availability, reduces the impact of aluminum and manganese toxicity, and enhances microbial activity, supporting better crop growth and soil health. Contact us to explore how H-Start can make a difference on your farm today.